In-lab Question 7. Select the Correct Metal Below.
3730 mL 565 mL 3165 mL 1 point is earned for the correct volume. The units of C are JC.

Computer Area Display Words Computer Lab Posters Classroom Computers Teacher Technology
Turn the hot plate to LOW and then go to the restroom.

. -NaCl - CaCl2 -FeCl3 -BaCl2 - PbCl2 The equation 2Als 3Br2l 2AlBr3s is an _____ reaction. Addition of NH 3 also known as NH 4 OH makes the solution basic and precipitates the hydroxide of Fe 3 rule 5. Lead II nitrate and sodium iodide solutions.
Energy Diagram for Reaction between Zinc Metal and Copper II Ion. Select the correct metal below. The water in the calorimeter rises to 3545 C.
Consider the redox reaction 1 and Figure 1 below. Place a watch glass on top of the flask and then quickly go to the restroom. Because HA is monoprotic.
You will be stressing these equilibria by adding products and reactants and observing the color changes that result. Select the correct metal below. Heat of Neutralization Mass g Temperature C Cups and cover from Part A 9623 NA NH 3 solution NA 2236 Cups cover and mixed solution 111884 NA Mixed Solution 102261 3134 In-Lab Question 8.
Note each specimen is stamped for identification. Select the correct metal below. Select the statements below that most closely agree with your observations.
1 Zn s Cu 2 aq Zn 2 aq Cu s Figure 1. Heat of Neutralization Mass g Temperature C Cups and cover from Part A 12711 NA NH 3 solution NA 2210 Cups cover and mixed solution 112711 NA Mixed Solution 100000 3220 In-Lab. If a precipitate forms put Y in the space that corresponds to the two solutions that were mixed.
After 5 minutes record your observations. The following properties are of minerals often found in igneous rocks. 1 mol HA 1.
Select all that apply The computer model of NH3 had a slightly smaller H-N-H bond angle than predicted by hybridizationIn general the molecules shape and model bond angles were close to the predicted values of 180 120 and 109 degreesThe computer model of H2O. 71017 430 AM Lab 4 InLab - Calorimetry Part C. Start studying the chem quiz 7 flashcards containing study terms like Which of the following ionic compounds is insoluble in water.
Half-fill a small test tube with copper II sulfate solution. In Part B of the calorimetry lab a piece of hot metal weighing 50850 grams immersed in hot water at 9765 C is placed into the Styrofoam calorimeter containing 4201 grams of water at 2455 C. Memorize flashcards and build a practice test to quiz yourself before your exam.
Add about 2 g of iron filings to the solution. Identify the correct label with its functionsA i Pulmonary vein - takes impure blood from body partB ii Pulmonary artery - takes blood from lung to heartC iii Aorta - takes blood from. One reactant zinc metal has a pair of electrons at a much higher energy level than an unfilled orbital in the other reactant copper II ion.
Heat of Neutralization Complete the following table. Heat of Neutralization Complete the following table. Use the mineral ID chart in your lab manual to help you identify each mineral.
Since you have 10 minutes until the solvent completely evaporates you may quickly go to the restroom. _ _ aluminum. Ask your TA to watch your experiment so you can go to the restroom.
HCl is added to the soluble nitrate salts of the metal ions rule 1 to precipitate AgCl rule 2. Investigating Some Exceptions to the Solubility Rules. The specific heat of water is 4184 JgC What is the value of qmetal.
A positive voltage will be observed when the red lead is attached to the metal with the more positive standard reduction potential and the black lead is attached to the metal with the more negative standard reduction. Chemical Reactions Lab Procedures. Select the correct metal below.
Lab 4 Online InLab - Calorimetry - CH_202 section 004 Fall 2020 _ WebAssignpdf. -oxidation-reduction and synthesis -oxidation-reduction only -synthesis. CO copper brass iron lead zinc Ni nickel AL aluminum Clamp the nickel specimen to one of the posts on the connector collar and set the connector collar on the glass container.
Sheet Metal Operations - 1 20 questions in 60 minutes Mock test for Mechanical Engineering preparation Free important questions MCQ to study RRB JE for Mechanical Engineering for Mechanical Engineering Exam Download free PDF with solutions. When the metal and water are mixed select all of the following that are true from CH 201 at North Carolina State University. Chemical Reactions Lab Procedures.
The CoCl 4 2-ion is an intense blue the color of the patterns on Delft china. Question 7 1 point For the galvanic cells examined in this lab which of the following statements isare select all that are correct true. Record your observations in Data Table B.
Obtain a set of 7 metal specimens. In-Lab Question 7. Each question enhances review by including a test-taking strategy and rationale for correct and incorrect answers.
A portion of the added NH 3 reacts with the HCl from the first separation to form NH 4 1 ammonium cation forming a buffer solution with the unreacted. Iron metal and copper II sulfate solution. 8 8 pts Question 9 Certain minerals are often found in different types of rocks.
The CoH 2 O 6 2 ion is pale pink. The absolute value of q metal is equal to the absolute value of q water They are both positive The absolute value of q metal is greater than the absolute value of q water They are both negative They have opposite signs The absolute value of q metal is less than the absolute value of q water426. Select the mineral that best fits the properties listed.
At the equivalence point moles OH added moles ofH consumed. B Based on the given information and your answer to part a determine the value of the concentration of the acid that should be recorded in the students lab report. NCLEX-RN Prep Plus by Kaplan 24th Edition Kaplans NCLEX-RN Prep Plus uses expert critical thinking strategies and targeted sample questions to help you put your expertise into practice and face the exam with confidence.
In Part C of the experiment students will use a spectrophotometer to calculate the equilibrium constant of bromothymol blue at three different. Nickel will be used as a reference for all measurements in part 1. The figure given below shows a schematic plan of blood circulation in humans with labels i to iv.
Δ T is the temperature change defined as Δ T T final - T initialHeat capacities depend upon the mass of the sample so the specific heat the amount of heat needed to raise the temperature of one gram of a substance by 1C is. In two of the unused wells of your well plate mix the solutions listed in Data Table B as you did for Part A. Where C water is the heat capacity of the water the amount of heat needed to raise the temperature of the water by 1C.

Molecular Geometry Worksheet Lab Activity Iteachly Com Molecular Geometry Lab Activities Classroom Science Experiments
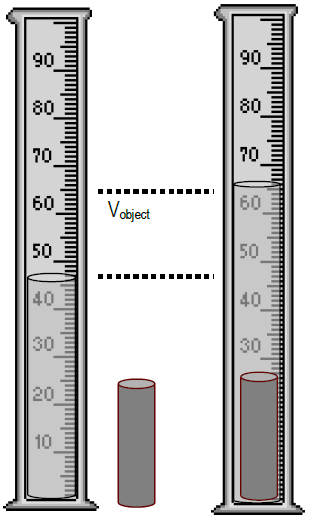
Lab 1 Density Determinations And Various Methods To Measure Volume

Tourmaline Ring Gold Ring Multi Tourmaline Ring Pink Tourmaline Gold Gemstone Ring Solitaire Ring Genuine Natural Tourmaline In 2021 Gold Gemstone Ring Pink Tourmaline Ring Gemstone Stacking Ring

20 Civil Engineering Standard Datas Interview Questions An Immersive Guide By Lceted Inst For Civil Engineers

Pin On New Year S Pregnancy Announcements

Rose Gold Fire Opal Ring Vine Leaf Engagement Ring Leaves Etsy Floral Engagement Ring Engagement Rings Opal Rose Gold Opal Ring

28 Handcrafted Alternative Non Traditional Engagement Rings Nontraditional Engagement Rings Vintage Engagement Rings Unique Engagement Rings

How To Clean Laboratory Glassware Lab Manager

Archived Quick Tip Revert To Classic Cricut Canvas Cricut Tutorials Cricut Tips
/laboratory-glassware-in-lab--measuring-flasks-and-cylinders-containing-chemicals-during-experiment-480810999-5b9fe8eec9e77c0050d32358.jpg)
Random Vs Systematic Error Definitions And Examples

Raw Amethyst Ring Pave Amethyst Band Alternative Engagement Etsy Amethyst Ring Engagement Wedding Ring Sets Unique Diamond Wedding Sets

Britiscientific Quiz Friday Feeling Planets
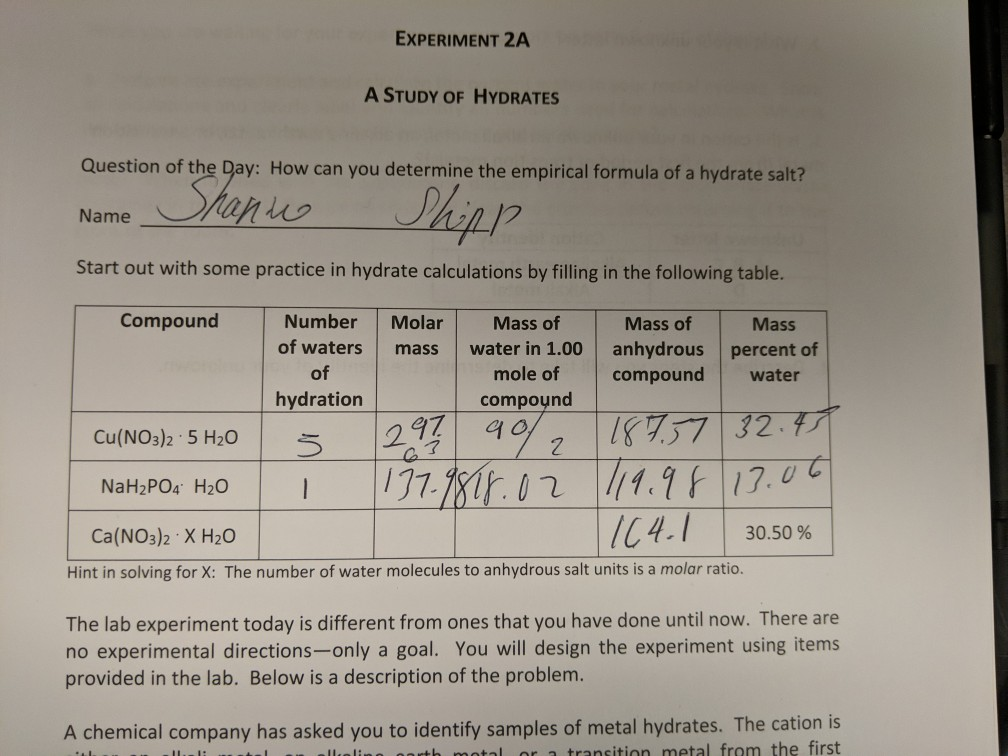
Solved Experiment 2a A Study Of Hydrates Question Of The Chegg Com

Scientific Method Scientific Inquiry Notes W Flip Book And Powerpoint Lesson Scientific Method Science Lessons Middle School Powerpoint Lesson

Cabi Shrunken Peacoat Jacket In 2021 Peacoat Jacket Striped Jacket Nike Windbreaker Jacket

Biology And Chemistry Student Resume Sample Student Resume Student Resume Template Resume Examples

Coshh Signs Dangerous Substances Packing And Labelling Information Notice Size 600 X 400mm Rigid Plastic Health And Safety At Work Health And Safety Coding

Comments
Post a Comment